
Lipids represent a large and diverse group of molecules, which can be divided into eight basic groups: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, sterol lipids, prenollipids, saccharolipids, and polyketides. Not only are lipids key components of cellular compartments, but they also store energy and have important signalling roles. However, diseases such as Alzheimer’s, cancer, diabetes and heart disease can be caused when these functions are affected. In order to study lipids, they must often be extracted first from tissues or cellular cultures and then quantified. There are various methods available for lipid quantification, ranging from state-of-the-art quantification using advanced technology to simpler benchtop solutions. But which technique is relevant for you?
Lipid Quantification Methods
Mass spectroscopy (MS)- based lipid quantification
Mass spectrometry (MS) is one of the main analytical techniques used in lipidomics, as it is highly sensitive, detects a vast number of lipid species and can also be used to quantify lipids. This technique has a strong dependence on equipment, requires higher sample amounts, can require tedious sample processing prior to the analysis and the technique may not be readily available to all laboratories.
Nuclear Magnetic Resonance
An alternative to MS-based lipid quantification and an altogether different approach for lipid profiling is proton nuclear magnetic resonance (1 H NMR) spectroscopy. The fundamental concept behind NMR spectroscopy is that all nuclei are electrically charged and have multiple spins. As the nuclei spin returns to its base position, the emission of energy occurs at the same frequency. This energy transfer matches with a signal, and the signal is detected in a number of ways to process and yield the same in the form of the corresponding nucleus’s NMR spectrum. A single internal standard is sufficient for quantitative analysis of all lipids owing to the linear response of the NMR signals of lipids, enabling this technique to be highly quantitative and reproducible. However again, this technique requires expensive equipment that may not be readily available to all laboratories and is in fact, less sensitive than MS since it requires a sample amount in the milligrams.
Infrared (IR) spectroscopy
Lipid samples can also be quantified by the determination of protein-to-lipid ratio by infrared spectroscopy. Since proteins and lipids show distinctive absorption bands, the IR spectrum can be used to estimate the protein-to-lipid ratio by dividing the relative intensity of the amide I protein band by that of carbonyl stretching of lipid-related ester bonds and therefore, quantify lipids. But again, this method requires equipment usually not available in research laboratories.
Sulfo-phospo-vanillin assay
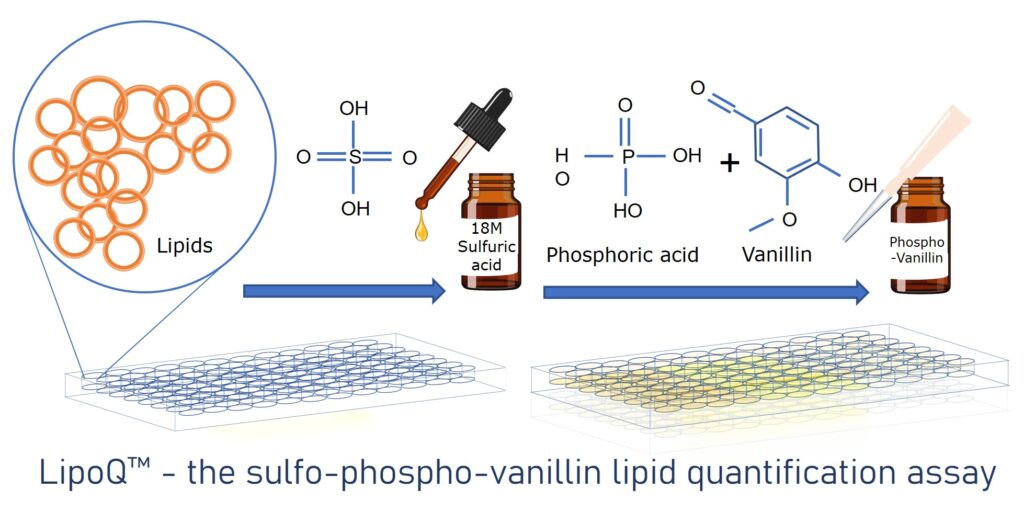
The sulfo-phospo-vanillin method measures the lipid content of unsaturated fatty acids within a sample. Unsaturated fatty acids within a sample will react with concentrated sulphuric acid, which in combination with phospho-vanillin will form a pink-colored solution. The intensity of the pink colour formed is determined by the total lipid concentration within the sample, enabling a reliable colorimetric assay that can be read using a plate reader. Cell Guidance Systems has developed the LipoQ™ lipid quantification kit based on this principle. This simple, benchtop top assay is amenable to a multiwell plate and results in a simple colorimetric readout, that does not require the use of equipment not usually available in research laboratories. For more information, visit the LipoQ™ product page.
IMAGE: Bigstock
Copyright Cell Guidance Systems
- 0 Comments
- exosome



