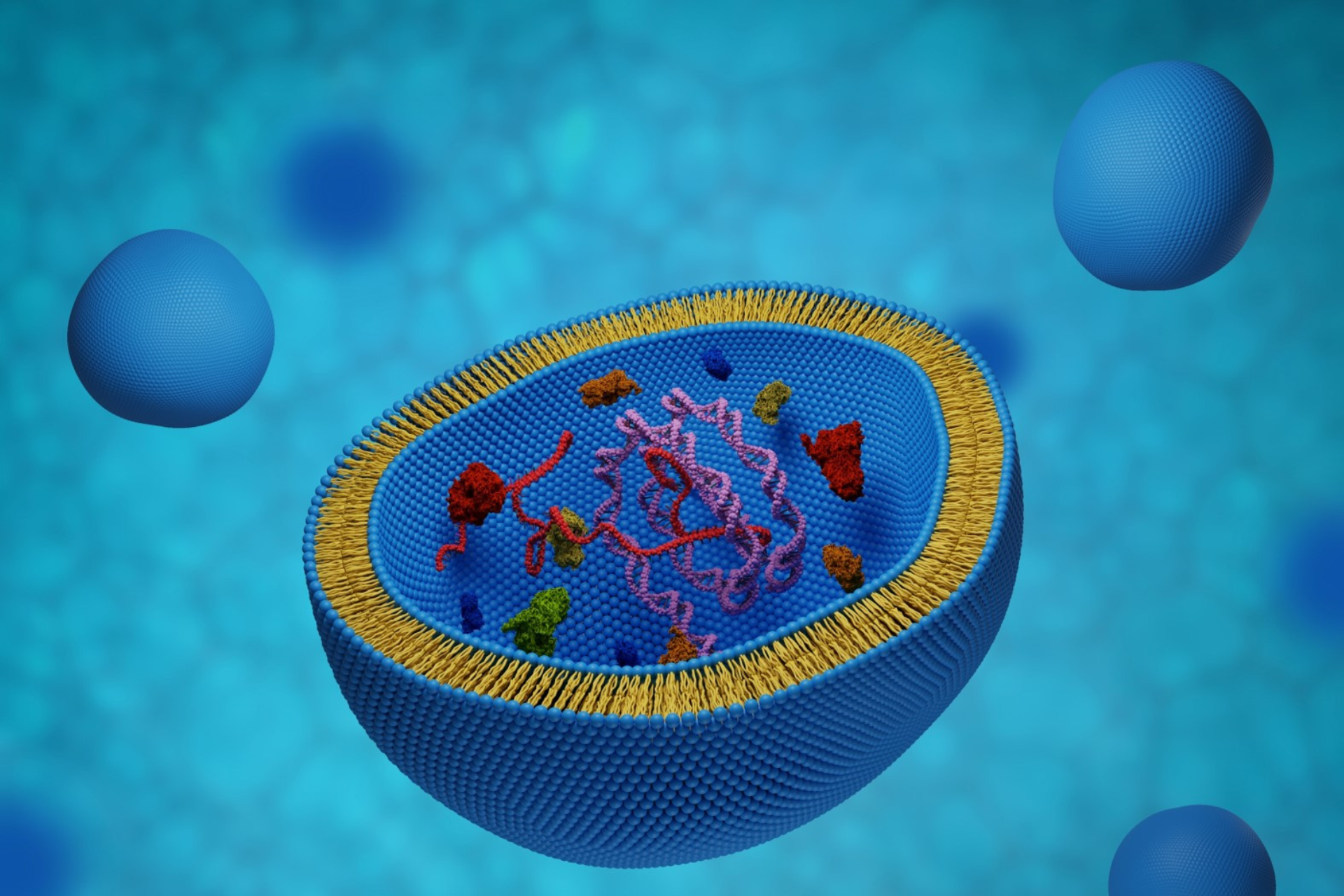
The recent emergence of genetic therapies has focussed attention on exosomes as a possible mechanism for their efficient delivery. Exosomes provide an efficient, natural mechanism for transferring RNA into cells. Exosomes are also durable, have low levels of immunogenicity and can be produced economically at scale. The biggest hurdle to the widespread adoption of exosomes as delivery vehicles is their low cargo-loading efficiency.
The delivery of RNA drugs is particularly challenging. In contrast to small molecules and protein drugs, a nucleic acid drug has to be delivered inside the cell to work. The few nucleic acid drugs that have been approved to date have relied on liposomes and viral delivery, notably adeno-associated virus (AAV).
AAV has the advantages that tropism (preference for particular cell types) allows targeting, and the persistence of AAV in infected cells allows a single dose to provide years of activity. Whilst this durability may be an advantage, it can also pose a hazard: A mechanism to turn off a drug’s activity when toxicity arises is important. Similarly, concerns about the oncogenic potential of AAV and immunogenicity have hampered enthusiasm for this drug delivery vehicle. Moreover, AAV manufacturing costs (typically $100,000 per dose) contribute to the multi-million dollar price of some of these therapies.
Liposomes are small, artificial vesicles that can be created from cholesterol and natural, non-toxic phospholipids. Liposomes are already widely used for the delivery of RNA vaccines. Lipid formulations have the advantage that they are inexpensive to manufacture at scale and the cargo is efficiently loaded. This cargo is delivered by merging liposomes with the cell’s lipid outer membrane. Although the efficiency of loading nucleic acids into liposomes is high, this delivery from liposome to cell has very low efficiency. Consequently, liposomes are far from ideal.
Similar to liposomes, exosomes form protective lipid spheres around cargo molecules. Compared to liposomes, exosomes are much more complex: For example, they contain a much larger variety of lipids and have characteristic proteins (such as tetraspanins) embedded in their walls. Importantly, exosomes merge with target cells much more efficiently than liposomes to deliver their cargo. However, the prior loading rate of specific cargo molecules into exosomes is very low.
If exosomes could be loaded as efficiently as liposomes, this would provide an efficient drug delivery system. Broadly speaking, there are three strategies being explored to load exosomes more efficiently:
Create artificial exosomes and load them during the synthesis process.
The challenge here is to generate a complex natural structure in an artificial process. Many types of artificial exosome strategies have been developed. These include hybrid structures that combine natural exosomes with pre-loaded liposomes. But significant challenges remain and these types of structures are not yet ready for clinical trials.
Improve loading during formation in cells
Cells naturally produce exosomes with a wide variety of cargo molecules. Increasing the proportion of exosomes carrying specific cargo molecules is another strategy being pursued. This may be achieved by modifications which would increase their efficiency of loading by the ESCRT complex during formation. However, this has been difficult to achieve.
Loading purified natural exosomes
A wide variety of techniques have been applied to load cargos into purified exosomes. These include techniques that have been used to deliver nucleic acids to cells such as electroporation, transfection, and the use of liposomes. It also includes techniques that would not be appropriate for cells, such as hypotonic dialysis, thermal shock and freeze-thaw cycling as well as the use of chemicals such as saponin. Each of these techniques has limitations and a highly efficient process that produces exosomes with an acceptable toxicity profile remains elusive.
IMAGE Shutterstock
- 0 Comments
- exosome



