
Glycosylation is the addition of glycan groups to proteins. This affects about 50% of proteins, including exosomal proteins, modulating their function. This impacts and reflects states of health and disease. As well as characterizing exosome glycosylation states for diagnostic purposes, glycosylation control strategies for therapeutic applications are in development.
As with all nanoparticles, exosomes have relatively large surface area-to-volume ratios. The heterogeneity of molecules present on their surface in healthy and disease states impacts the specific ways exosomes circulate, establish contact and reprogramme target cells. This feature can be exploited for use as a disease biomarker or as the target of surface engineering efforts to endow exosomes with additional functionality and expand their use in diverse therapeutic areas.
Glycans are simple sugars linked glycosidically. They are important metabolic and structural components in biological systems. These carbohydrate-based polymers are also recognised for their function as information carriers in complex molecular recognition events to modulate cellular functions. Aberrant glycosylation is a common feature of disease.
Many heavily glycosylated glycoconjugates and repeating glycosaminoglycan chains have been identified in exosomes that exert key physiological functions. High-content screening to examine the role of glycosylation in the interaction of exosomes with cells found a broad range of responses with different preferences between cell types for glycosylation patterns, glycan sequences, connections and lengths. This suggests glycans provide a key mechanism by which tuning of uptake is controlled.
These findings also suggest the potential of using exosomal glycans and/or their glycosylation profiles for disease diagnosis. For example, prostateâ€specific antigen (PSA), a widely used biomarkers for prostate cancer, is found at high levels on the surface of exosomes isolated from the urine and blood of prostate cancer patients. Specific glycosylation patterns of PSA correlate with disease state significantly better than the traditional PSA test. Similarly, upregulation of Oâ€GlcNAcylation, αâ€2â€HSâ€glycoprotein and highly glycosylated CD24 glycoprotein on exosomes can serve as a basis for detecting other cancers.
The glycosylation profile of exosomes can also be used as a way to purify exosomes from body fluids. Lectinâ€glycanâ€binding microarray has recently emerged as a tool for selective exosome isolation. This technique may address some of the challenges in the recovery and purification of cellular exosomes in sufficiently large quantities required for clinical applications.
Glycoengineering may be developed into a toolkit to manipulate hallmark features of exosome biology, such as cellular secretion and recognition. Glycoengineering of exosomes has potential to activate the immune system against tumours, manipulate cargo recruitment, and provide targeted drug delivery.
The structures of glycans are complicated and profiling/characterising global glycosylations of a certain target type of exosome, cell or even tissue, is a huge challenge. Moreover, there is no sequence relationship between glycans and their conjugates other than initial attachment sites. The amount of different glycoconjugates to exosome surfaces from various sources is also uncertain.
Whilst still at an early stage, the field of exosome glycomics is becoming increasingly well-established but the complexity of glycan biochemistry in exosome biology may be leading to a slower pace of research compared with the other major biomolecules such as proteins, lipids and nucleic acids. As the importance of glycosylation becomes more widely appreciated, we are likely to see significant applications emerging.
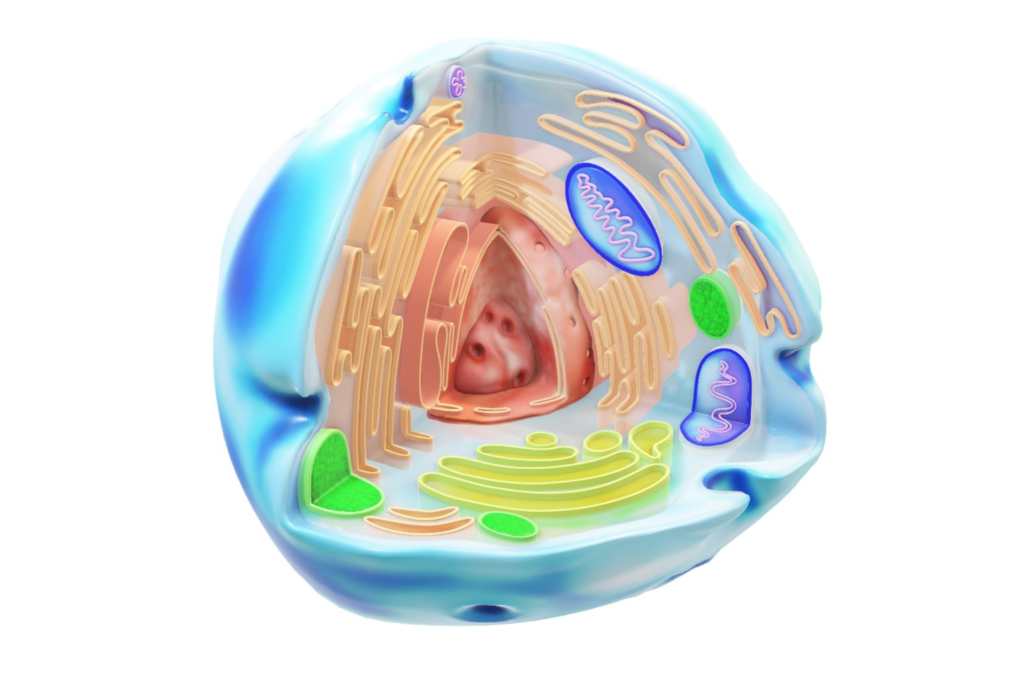
IMAGE Glycosylation occurs mainly at the endoplasmic reticulum (orange) and golgi bodies (yellow)
- 0 Comments
- exosome



