Abstract
Exosomes are extracellular vesicles secreted by most eukaryotic cells and participate in intercellular communication. The components of exosomes, including proteins, DNA, mRNA, microRNA, long noncoding RNA, circular RNA, etc., which play a crucial role in regulating tumor growth, metastasis, and angiogenesis in the process of cancer development, and can be used as a prognostic marker and/or grading basis for tumor patients. Hereby, we mainly summarized as followed: the role of exosome contents in cancer, focusing on proteins and noncoding RNA; the interaction between exosomes and tumor microenvironment; the mechanisms that epithelial-mesenchymal transition, invasion and migration of tumor affected by exosomes; and tumor suppression strategies based on exosomes. Finally, the application potential of exosomes in clinical tumor diagnosis and therapy is prospected, which providing theoretical supports for using exosomes to serve precise tumor treatment in the clinic.
Introduction
Exosomes, with a size range of 40–160 nanometers in diameter (averaging 100 nanometers), are a subset of extracellular vesicles (EVs) surrounded by a lipid bilayer membrane and secreted by most eukaryotic cells,1 Identified as early as in late 1980s, exosomes were originally and simply considered as cellular waste products.2 However, with the development of research methodologies and techniques, people now have realized that exosomes represent a novel mode of intercellular communication and contribute to a wide range of biological processes in health and disease including cancer.3 The biological function of exosome relies on its bioactive cargos, such as lipids, metabolites, proteins and nucleic acids,4,5,6,7 which can be delivered to the target cells. Growing evidence suggests that tumor-derived exosomes (TEXs) play critical roles in cancer. Exosomes and their cargos may serve as cancer prognostic marker, therapeutic targets or even as anticancer drug‐carrier.8 In this review, we endeavor to summarize the bioactive exosomal contents focusing on proteins and noncoding RNAs, clarify the crosstalk of exosome with tumor microenvironment (TME), elucidate the underlying mechanism of affected epithelial-mesenchymal transition (EMT), invasion and migration affected by exosomes, and discuss the future tumor suppression strategies based on exosomes.
Exosomes biogenesis and isolation
Biogenesis
Exosomes are originated from the endocytic pathway.9 A typical process of exosomes formation comprises the following steps (Fig. 1): (i) the cytoplasmic membrane invaginates to form an early secretory endosome; (ii) followed with the payload sprouts inward to form intraluminal vesicles (ILVs) contained within the endosome, which termed a multi-vesicular bodies (MVBs) biogenesis; (iii) then, the late endosomes maturation by acidification; (iv) and eventually extracellular release of ILVs as exosomes by fusion with the plasma membrane.10 It is known that the endosomal sorting complex required for transports (ESCRTs) mechanism plays an important role in the process of MVBs and ILVs biogenesis. The components of ESCRTs (containing ~20 proteins) comprises four complexes, termed ESCRT-0, -I, -II, and -III, with associated proteins, including ALIX (Apoptosis-linked gene 2-interacting protein X, encoded by PDCD6IP), VTA1(Vesicle Trafficking 1), VPS4 (Vacuolar protein sorting-associated protein 4), and TSG101 (Tumor susceptibility gene 101 protein).11,12 During the process of MVBs biogenesis, the ESCRT-0 complex is recruited by ubiquitinated cargo, the major pathway-specific signal in MVBs biogenesis, to the endosomal membrane; the ESCRT-I and -II components make membrane deformation into buds and isolate the payload; and the ESCRT-III complex separates the vesicles from the cytoplasmic membrane, subsequently.13,14 In addition, other ESCRTs-independent mechanisms have been found to affect the formation of exosomes, such as neutral sphingomyelinase 2-dependent pathway, heterogeneous nuclear ribonucleoprotein-dependent pathway, miRNA post-transcriptional 3′end modification and RNA induced silencing complex related pathway.
Fig 1
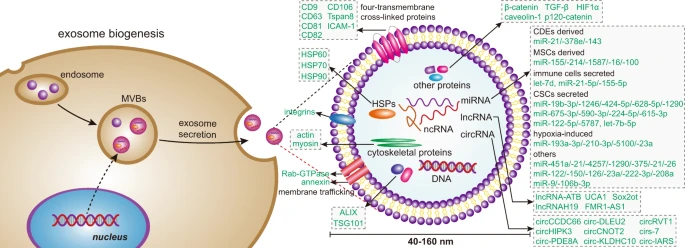
Exosome biogenesis and its contents. Exosomes originated from MVBs, which contain many kinds of proteins, such as membrane transporters, HSPs and so on. In addition, it also contains a lot of noncoding RNA, including miRNA, lncRNA, and circRNA. These contents play an important role in the development of tumor. MVBs multi-vesicular bodies, CDEs cancer-associated fibroblast-derived exosomes, MSCs mesenchymal stromal cells, CSCs cancer stem cells
Exosome and cancer-associated fibroblasts
CAFs are the main cellular components of TME in most solid cancers.71 CAF-derived exosomes (CDEs) are one of the key factors of oncogenic transformation.72 CDEs promotes the growth of cancer cells by inhibiting mitochondrial oxidative phosphorylation, thereby increasing glycolysis and glutamine dependent reduction carboxylation in cancer cells.71 In breast cancer patients, the expression levels of miR-21, miR-378e, and miR-143 in CDEs were higher than those in NFs. Breast cancer cells transfected with these three miRNAs could promote the stemness and EMT of these cells.73 CDEs not only enhance cancer growth, but also promote drug resistance and tumor metastasis.74 For example, CDEs could promote neoplastic angiogenesis and tumor development in CRC, and could also induce the dedifferentiation of cancer cells through the Wnt pathway, thereby promoting the chemical resistance of CRC.75,76 Studies have found that CAFs were inherently resistant to gemcitabine (GEM, chemotherapy standard for pancreatic ductal adenocarcinoma). CAF exposure to GEM significantly increased the release of exosomes, thereby increasing cell proliferation and survival of recipient epithelial cancer cells.77 In addition, CAFs produce exosomes with high level of TGF-β1,78 which is capable to induce metastatic activity of cancer cells by regulating the expression of lncRNA.79 Yu. et al. reported that TGF-β1 was top one highest level of cytokine secreted by CAFs, which was essential for CAFs-induced EMT and metastasis in breast cancer cells. CAF-derived conditional medium significantly increased the HOTAIR expression to promote EMT of breast cancer cells. However, this could be abrogated by treatment with TGF-β1 inhibitor SB451332 or Pirfenidone. Moreover, depletion of HOTAIR inhibited CAFs-induced tumor growth and lung metastasis in MDA-MB-231 orthotopic animal model.80 In HCC, overexpression of lncRNA-ATB further promoted metastasis of hepatoma cells by inducing EMT and invasion.81
Exosomes and cancer stem cells
CSCs, also known as cancer-initiating cells, are a small subset of heterogeneous cells present in tumor tissue. CSCs have unlimited self-renewal and diversification potential and play an important role in tumor initiation, recurrence, metastasis, and therapeutic resistance.82 As information carriers, exosomes are involved in the transformation between non-CSC and CSC as well as the maintenance of CSC homeostasis and its mechanism.23 lncRNA FMR1-AS1 in exosomes could maintain the dynamic interconversion state of CSCs by activating TLR7-NFκB signaling.83 The exosomes derived from CSC transferred miR-19b-3p to clear cell renal cell carcinoma cells, and then initiated EMT to promote tumor metastasis.84 In contrast, p120-catenin in exosomes secreted by HCC (hepatocellular carcinoma) cells inhibited the proliferation, metastasis, and expansion of HCC CSCs.85 Exosomes are also important participants in the resistance of CSCs, which involving multiple mechanisms, including enhanced DNA repair efficiency and anti-apoptotic capacity, slow cell cycle progression, drug efflux, and expression of detoxifying enzymes.86 For example, in PC, BxR-CSC (GEM-resistant BxPC-3 cells)-derived exosomes induced GEM resistance, inhibited GEM-induced cell cycle arrest, and antagonized GEM-induced apoptosis. It also promoted tube formation and cell migration of BxS (GEM-sensitive BxPC-3 cells) and PANC-1 cells.87 In breast cancer, RAB27B promoted exosomes transfer from stromal cells to breast cancer cell with the transfer of exosomal 5′-triphosphates which activated the RIG-I (retinoic acid-induced gene 1 enzyme) signal in target cells, thereby activating IRDS (Interferon-Related DNA Damage Resistance Signature) genes, in parallel activation of the NOTCH3 pathway to regulate the expansion of therapy-resistant tumor-initiating cells known as CSCs.88
Exosome and mesenchymal stem cells
MSCs are considered to be one of the most promising seed cells in tissue engineering because of their easy availability and pluripotency characteristics of adipocytes, osteoblasts, cardiomyocytes, and neurons.89 Exosomes derived from MSCs in the TME facilitate the conversion of non-CSC to CSC. Exosomes released by MSCs confer colorectal stem cell phenotypes by activating the Wnt signaling pathway, including tumor spheroid formation in vitro and tumorigenicity in vivo, increasing the percentage of CSCs, and activating ERK1/2 (extracellular signals to regulate kinase 1/2), thus, promoted tumor growth and progression.90 In tumors, MSC-derived exosomes regulate tumor markers and help tumor progression by delivering special miRNA to neighboring cells. For example, bone marrow-derived MSCs released exosomes containing miR-214 through CaMKII silencing to inhibit oxidative stress injury in CSC, thus helping tumor progression.91 Exosomes from tumor-associated MSCs contained high levels of miR-155, which, after being taken up by atypical teratoid/rhabdoid tumor cells, could cause tumor suppressor genes SMARCA4 (direct target genes of miR-155) inhibition, and enhanced atypical teratoid/rhabdoid tumor migration ability.92 In addition, glioma-associated human MSCs, a potential new matrix component in glioma, could drive the invasibility of glioma stem cells (GSCs) and have been identified as a new therapeutic target in glioma.93 miR-1587 in glioma-associated human MSCs derived exosomes promoted proliferation and clonal formation in GSCs by down-regulating the tumor-suppressive nuclear receptor corepressor NCOR1 in GSCs.94 However, it is interesting that MSC exosomes also contain anti-angiogenic miRNAs, such as miR-16 and miR-100, which inhibit angiogenesis by targeting vascular endothelial growth factor in breast cancer cells in the TME.95,96,97 In addition, MSC-derived exosomes also play an important role in tissue repair and inflammatory response.98
Exosome and tumor microenvironmental immune cells
The tumor microenvironmental immune cells mainly include myeloid cells (tumor-associated macrophages, dendritic cells (DCs), myeloid-derived suppressor cells, etc.) and lymphocytes (T and B cells).99 Tumor cell-derived exosomes are a good source of cellular components that stimulate the immune response, such as alarmins (mRNA, transmembrane proteins including CD9, CD63, and CD81, HSPs, major histocompatibility complex I molecules) and tumor-associated antigens.100 The antigen-presenting and immune-stimulating properties of TEXs enable them to trigger antitumor responses and contribute to the recruitment and reconstruction of tumor microenvironmental components.101 For example, TEXs derived from oral squamous cell carcinoma and histiocytic lymphoma could induce immunosuppression through the CD95 (Fas) receptor and FasL+ exosomes signaling on activated CD8+ T cells.102,103 PC-derived exosomes switched the differentiation of macrophages to the M2 phenotype, thereby promoting the immunosuppression and metastasis of HIF-1α or HIF-2α.104,105 Exosomes derived from Lewis lung cancer or 4T1 breast cancer cells prevented the differentiation of myeloid precursor cells to CD11c+ DCs and induced apoptosis.106 Under hypoxic conditions, tumor cells released exosomes with TGF-β, which could also promote Treg cell activity and inhibited NK cell cytotoxicity to form an immunosuppressive environment.107,108 Tumor microenvironmental immune cells also produce exosomes. Treg cells prevented the maturation of antigen-presenting cell by expressing CTLA-4, or produced inhibitory cytokines and adenosine anti-tumor immunity to participate in tumor development and development.109 Treg-derived exosomes had also been implicated in immunosuppressive functions.110,111 Treg cell exosomes miRNA (Let-7d) strongly inhibited Th1 cell activity by inhibiting COX-2-mediated IFN-γ production.112 Macrophages are the major immune cells that regulate inflammation in the TME, including two broad phenotypes, M1 and M2. M1 macrophages can kill viruses and bacteria by secreting IL-12 and IL-23 inflammatory factors. M2 macrophages can promote blood vessel formation and tumor growth and metastasis.113 In CRC, exosomes secreted by M2 macrophages highly expressed miR-21-5p and miR-155-5p to regulate the migration and invasion of CRC cells.114
Exosomes affect EMT, invasion and migration
Exosome signaling can induce the production of CSCs through EMT in the TME and generate favorable treatment resistance conditions.115 EMT refers to the biological process by which epithelial cells are transformed into cells with mesenchymal phenotypes through specific programs. EMT regulation requires a complex transcription mechanism, mainly composed of developmental transcription factors, which can be divided into three categories: SNAIL family of zinc-finger transcription factor SNAIL/SLUG, ZEB1/ZEB2 ZEB (zinc-finger e-box binding homogenic box) family, and TWIST1/TWIST2 family.116 In addition to transcriptional factor, EMT regulation also involves multiple noncoding RNA inducers, including miRNAs and lncRNAs.117,118,119 TEXs are likely to carry these inducer molecules to facilitate the EMT process. In examining the biological function of exosomes from highly metastatic lung cancer cells and human advanced lung cancer, researchers found that those exosomes can increase Vimentin expression, promote EMT, invasion and migration of target cells.120 Exosomal miR-106b-3p from CRC cells was found abundant in metastatic CRC patients’ serum and correlated with poor prognosis. Further research demonstrated that exosomal miR-106b-3p can increase lung metastasis of CRC cells via Vimentin, N-cadherin upregulation and E-cadherin down-regulation by targeting DLC-1 (Deleted in Liver Cancer-1) gene.121 In pancreatic ductal carcinoma(PDAC), lncRNA-Sox2ot was identified from highly invasive PDAC cells, and plasma exosomal Sox2ot expression was closely associated with TNM stage and overall survival rate of PDAC patients. Exosomal Sox2ot works as ceRNA who can competitively bind with the miR-200 family to regulate SOX2 expression, which increased EMT and stem cell-like properties, resulted in promotion of invasion and metastasis of PDAC.122 It was reported that PDAC cell-derived exosomal circ-PDE8A acts as a ceRNA for miR-338 to regulate MACC1 and stimulates invasive growth via the MACC/MET/ERK or AKT pathways. Moreover, exosomal circ-PDE8A expression in plasm of PDAC patients was related with progression and prognosis, thus suggest it may play an important role in tumor invasion, could be a potential marker for PDAC diagnosis or progression.
123 TEXs can carry proteins including TGF-β, caveolin-1, HIF1α, and β-catenin, which enhanced invasiveness and the ability of target cells to migrate, and helped matrix remodeling and niche formation.124 Exosomes derived from gastric cancer cells could induce the differentiation of human umbilical cord-derived MSCs to CAFs by transferring TGF-β and activating TGF-β/Smad pathway, which assisting tumor niche formation.125,126 It has been demonstrated that the presence of caveolin-1 in prostate cancer-derived exosomes could potently induce CSC phenotype and EMT process via NF-κB signaling.127 Exosomes containing high expression of caveolin-1 could promote migration and invasion of tumor cells lacking caveolin-1.128 Aga et al. detected endogenous HIF1α in nasopharyngeal carcinoma-derived exosomes and found that exosomes treatment of LMP1 (latent membrane protein 1), a principal oncoprotein of EBV (Epstein-Barr virus), obviously enhanced the levels of HIF1α in exosomes and increased migration and invasiveness of EBV-negative nasopharyngeal carcinoma cells.129 Additionally, HCC cells released exosomes contained β-catenin, and Vps4A, a classical tumor suppressor, could downregulate exosome release of β-catenin and inhibit EMT and motility of HCC cells via inhibiting β-catenin signaling.130 The CRC cells secreted exosomes could transfer mutated β-catenin to recipient cells activating Wnt signaling and enhance tumor burden in xenograft mice models.
131 TGF-β and WNT/β-catenin signaling pathways are key regulators in EMT.132,133,134 Exosomes derived from HCC cells could mediate EMT through activating TGF-β/Smad signaling pathway, inducing a decrease in E-cadherin expression, but an increase in Vimentin, which resulted in promoted migration and invasion of target cells.135 The TGF-β1/Smads pathway was an important mechanism for bone marrow-derived MSCs to induce EMT in breast cancer cells.136 The Wnt/β-catenin signaling pathway and Wnt activity are associated with ovarian cancer classification, EMT, chemotherapy resistance, and poor prognosis. In addition, the ERK protein subfamily made a huge contribution to EMT and was overexpressed and overactive in various types of cancers.137 Pancreatic stellate cells, typically activated in pancreatic ductal adenocarcinoma, -derived exosomes increased the expression of ERK1/2 (extracellular signals to regulate kinase 1/2) in PDAC cells, which leading to promote cell migration, EMT, and enhance matrix metalloproteinase‑2/9 activity.138 Highly metastatic HCC MHCC97H cells-derived exosomes could communicate with low metastatic HCC cells and subsequently enhanced its migration, invasion, chemotaxis, and EMT by ERK signaling.139 It is very interesting that hypoxia is a very important factor involving in exosomes regulated EMT. Exosomes derived from hypoxic bone marrow-derived MSCs mediated transfer of miRNAs, including miR-193a-3p, miR-210-3p and miR-5100, from bone marrow-derived MSCs to lung cancer cells, increased the expression of total and phosphorylated STAT3, thus promoted invasion of lung cancer cells by activating STAT3 signaling-induced EMT.140 Hypoxia-induced CRC-derived exosomes, containing miR-23a, which could lead to the down-regulation of PDH1/2 when applied to CRC cells, thus eventually upregulated HIF-1α, an independent activator of EMT.141
Exosome-based tumor suppression strategies
Exosomes as drug carriers-targeted inhibition of tumor cells
In order to improve the effectiveness of cancer treatment, we urgently need to accurately deliver drugs to tumor cells. Clinically, nanotechnology-based drug delivery systems are one of the most promising tools to achieve this goal. Using its natural delivery capabilities, exosomes have been successfully used as drug and functional RNA delivery vectors in cancer treatment.142 Exosomes can be absorbed by cells and can stably transfer drugs, such as therapeutic miRNAs and proteins143 (Fig. 3). Compared with liposome nanomaterials, metal nanomaterials, and polymer nanomaterials, exosomes as carriers can overcome the shortcomings of poor bioavailability and reduce non-targeted cytotoxicity and immunogenicity.144,145 And exosomes contain transmembrane and membrane anchoring proteins, which enhance endocytosis and thus promote the transfer of their contents.146 For instance, Kim et al. found that paclitaxel-loaded macrophage-derived exosomes significantly increased cell uptake in 3LL-M227 mouse Lewis lung cancer cell line, compared to paclitaxel-loaded liposomes.147,148 Compared with free drugs in animal tumor models, exosome-mediated chemotherapy delivery can enhance drug effects. For example, the antimitotic chemotherapy drug paclitaxel could be loaded into exosomes by sonication. And these loaded exosomes were 50 times more cytotoxic to drug-resistant cancer cells in vitro than free paclitaxel.148 Additionally, compared with free drugs, exosome-based delivery platform can greatly reduce side effects. Studies have shown that exosomes coated with different chemotherapeutic drugs were transported to the tumor tissues of mice and could inhibit tumor growth, but no equivalent side effects of free drug have been observed.149 The use of engineered exosomes containing miR-21 sponge constructs had the potential to downregulate the expression of miR-21 in glioma cell lines U87-MG and C6, thereby up-regulating the target genes PDCD4 and RECK of miR-21 and preventing their malignant behavior.150 Recent studies have shown that exosome surface modification is performed using oligonucleotide binding methods. Such cargo may not only potentially alter cell function, but also alter cell-to-cell transport.151 Triple-negative breast cancer is one subtype of breast cancer and with the most metastatic and recurrent characteristic. Li et al. modified the surface of the exosomes with a peptide targeting mesenchymal-epithelial transition factor gene (c-Met), a tyrosine kinase receptor for hepatocyte growth factor or scatter factor, which is overexpressed on triple-negative breast cancer cell surfaces.152 These engineered exosomes showed significantly improved cellular uptake efficiency and antitumor efficacy of doxorubicin.
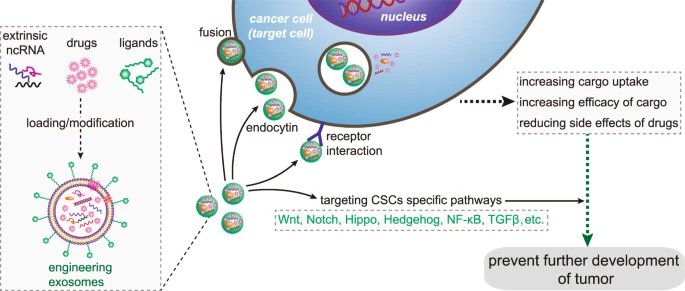
Precisely targeting tumor with engineering exosomes as delivery carrier. Exosomes are carriers with natural delivery ability, which have the characteristics of precisely targeting and high bioavailability. After being loaded into exosomes, anticancer drugs and/or extrinsic ncRNA can directly target cancer cells or CSCs specific pathways, and prevent the further development of tumors. Additionally, the surfaces of exosomes can also be modified with the ligands corresponding to receptors overexpression on cancer cell surfaces, which improving cellular uptake efficiency of exosomes by cancer cells. ncRNA noncoding RNA, CSCs cancer stem cells
Exosomes can also affect CSCs by targeting CSC-specific signaling pathways, such as Wnt, Notch, Hippo, Hedgehog, NF-κB, and TGF-β pathways. These pathways are of great significance for maintaining a series of biological functions, like self-renewal, differentiation, and tumorigenesis of CSCs. Selective targeting of CSCs via the above pathway using exosome loading inhibitors (miRNA or siRNA) is considered to be achievable.86 Lymphoma side population cells outputted Wnt signaling pathway agonist Wnt3a to neighboring cells through exosomes, thereby regulating the number balance of tumor cell population.154 Existing results have shown that exosomal Wnt from fibroblasts could induce dedifferentiation of cancer cells to promote chemotherapy resistance in CRC, and suggesting that interference with exosomal Wnt signaling could help improve chemosensitivity and treatment window.
Anti-tumor vaccine using exocrine system
TEXs have a dual effect on the immune system, i.e., immunosuppressive or immunostimulatory effects. Numerous research have shown that TEXs can interfere with the maturation of DCs, weaken the activation of NK cells, induce suppressor cells of myeloid origin, and transform macrophages into tumor-promoting phenotype.155,156,157 The activated CD8+ effector T cells in the circulation system of cancer patients were induced apoptosis by TEXs, which was one of many immunoinhibitory mechanisms of TEXs and suppressed patient’ general immune system.72 Exosome as a carrier for delivery products can initiate antitumor immune responses with significant therapeutic effects on tumor progression.158 In mouse model with melanoma, mice were treated with α-galactosylceramide/ovalbumin-loaded exosomes, which induced an early T cell response and eventually slowed tumor growth compared to the control group.143 Abundant alpha fetoprotein in exosomes produced by in vitro cultured HCC could stimulate the antigen-presenting function of DCs, stimulate the proliferation of CD8+ T cells, regulate the secretion of inflammatory cytokines (reducing IL-10 and TGF-β secretion and increasing secretion of IFN-γ and IL-2), and enhance immune-induced apoptosis.159,160,161 According to the study of Xie et al., a vaccine developed by exosomes was effective in antitumor immunity. In their study, exosomes from MM (multiple myeloma) cells were used to stimulate anti-tumor immune responses and generate prophylactic immunity in MM cell lines.162,163 TEXs recovered and enriched from patient sera may well provide an optimized, individual-specific source of antigen for DCs vaccination.164 How to make full use of the advantages of TEXs, and bypass their disadvantages to regulate tumor immunity needs further research, which has great potential in the application of cancer targeted therapy
Conclusions and perspectives
Cancer-related exosomes are produced by cancer cells, CSCs or tumor microenvironment associated cells. They contain numerous categories of contents such as proteins, DNA, mRNA, miRNA, lncRNA, and circRNA. Some of them act as biomarkers, we can take this advantage for cancer early detection, early diagnosis, prognosis prediction, and therapeutic efficacy evaluation. Some of the contents act as mediators of signal transduction between cancer cells with cancer cells, or cancer cells with tumor microenvironment associated cells, and contribute to tumor development, invasion, metastasis, and drug resistance. Based on this, we can develop new strategies relying on engineered exosomes carrying with tumor-suppressing proteins, nucleic acid components or targeted drugs function as precision medicine.
Although there have been many studies trying to clarify the molecular mechanism of how exosomes are produced, endocytosis and play biological roles in tumor progression, more efforts are still needed to further elucidate these problems to make the diagnostic and therapeutic potential of exosomes a clinical reality. We believe that with the continuous research work, in the near future, we may make full use of the advantages of exosomes as natural carriers and bypass the shortcomings. Great progress will be made in cancer treatment strategies based on exosomes, for the great goodness of vast cancer patients.



